The Present Scenario About Dolomite
Dolomite at present time, does not form
on the surface of the earth; yet massive
layers of dolomite can be found in
ancient rocks. That is quite a problem
for sedimentologists who see sandstones,
shales and limestones formed today
almost before their eyes.
DOLOMITE is a double carbonate of calium
and magnesium, CaCO3, MgCO3. The mineral
was first identified by Count Dolomien
in 1791 and named after its discoverer.
It is of sedimentary origin and is
supposed to have been formed due to
chemical action of sea-water containing
high percentage of magnesia, on limestone.
Theoretically, Dolomite Contains
| CaCO3 |
54.35% |
 |
| MgCO3 |
45.65% |
In Other Words, Dolomite Contains
| CaO |
30.4% |
 |
| MgO |
21.7% |
| CO2 |
47.9% |
In nature, considerable variations in
the composition of dolomite relating to
lime and magnesia percentages are found.
When the percentage of CaCO3 increases
by 10% or more over the theoretical
composition, the mineral is termed 'calcitic
dolomite', 'high-calcium dolomite' or
'lime-dolomite'. With the decrease in
percentage of MgCO3, it is called 'dolomitic
limestone'. With the variations of MgCO3
between 5 to 10%, it is called 'magnesian
limestone', and upto 5% MgCO3 or less it
is taken to be limestone for all
purposes in trade and commercial
parlance.
Dolomite usually contains impurities,
chiefly silica, alumina and iron oxide.
For commercial purposes, the percentage
of combined impurities should not go
beyond 7% above which, it becomes
unsuitable for industrial use. It is
then used only for road ballasts,
building stones, flooring chips etc.
| Hardness |
3.5-4 |
| Associated |
include calcite sulfide ore
minerals fluorite barite quartz
and occasionally with gold |
| Minerals Chemical/Typical
composition |
white |
| Color |
often pink or pinkish and
can be colorless, white, yellow,
gray or even brown or black when
iron is present in the crystal |
| Characteristics |
Unlike calcite, effervesces
weakly with warm acid or when
first powdered with cold HCl |
| Luster |
pearly to vitreous to dull |
| Field Indicators |
typical pink color, crystal
habit, hardness, slow reaction
to acid, density and luster |
Dolomite Packing
All grades of Gilsonite are available in various types of packaging:
1. 1 Ton Jumbo bags
2. 25kg package for powder
Dolomite Physical Characteristics
| Hardness |
3.5-4 |
| Specific gravity |
2.86 (average) |
| Cleavage |
|
| Color |
Often pink or pinkish and can be colorless, white, yellow, gray or even brown or
black when iron present in the crystal. |
| Density |
|
| Diaphaniety |
|
| Fracture |
Conchoidal |
| Crystal Habits |
Include saddle shaped rhombohedral twins and simple rhombs some with slightly
curved faces, also prismatic, massive, granular and rock forming. Never found in
scalenohedrons. |
| Luminescence |
|
| Luster |
Pearly to vitreous to dull |
| Streak |
White |
| Synonym |
|
| Transparency |
Crystals are transparent to translucent |
| Crystal System |
Trigonal; bar 3 |
| Cleavage |
Perfect in three directions forming rhombohedrons. |
| Other Characteristics |
Unlike calcite, effervesces weakly with warm acid or when first powdered with
cold HCl. |
| Associated Minerals |
Include calcite, sulfide ore minerals, fluorite, barite, quartz and occasionally
with gold |
| Notable Occurrences |
Many localities throughout the world, but well known from sites in Midwestern
quarries of the USA; Ontario, Canada; Switzerland; Pamplona, Spain and in Mexico
|
| Best Field |
Typical pink color, crystal habit, hardness, slow reaction to acid, density and
luster |
Dolomite Chemical Analysis
| Chemical Analysis |
% |
| SiO2 |
------ |
| Al2O3 |
0.04 |
| Fe2O3 |
0.024 |
| TiO2 |
N.D |
| CaO |
32.218 |
| MgO |
20.179 |
| Na2O |
------ |
| K2O |
------ |
| Insoluble |
0.094 |
| Na2CO3 |
------ |
| Loss |
47.33 |
| Total |
99.885 |
Dolomite Habits
Crystalline - Coarse - Occurs as
well-formed coarse sized crystals.
Massive - Uniformly indistinguishable
crystals forming large masses., Blocky - Rhombohedral - Crystal shape resemb les
rhomohedrons.
Associated Minerals include albite,
anatase, calcite, chlorite group,
fluorapatite, fluorite, galena,
gmelinite, marcasite, molybdenite,
pyrite, quartz, rutile, siderite and
sphalerite
Crystal habits include saddle shaped
rhombohedral twins and simple rhombs
some with slightly curved faces, also
prismatic, massive, granular and rock
forming. Streak is white.
The Dolomite Group of Minerals
The Dolomite Group is composed of
minerals with an unusual trigonal bar 3
symmetry. The general formula of this
group is AB(CO3)2, where A can be either
calcium, barium and/or strontium and the
B can be either iron, magnesium, zinc
and/or manganese.
The structure of the Dolomite Group is
taken from the Calcite Group structure.
The Calcite Group structure is layered
with alternating carbonate layers and
metal ion layers. The structure of the
Dolomite Group minerals is layered in
such a way that the A metal ions occupy
one layer which is followed by a
carbonate layer which is followed by the
B metal ion layer followed by another
carbonate (CO3) layer, etc. The layering
looks like this:
|A|CO3|B|CO3|A|CO3|B|CO3|A|... This
ordered layering of different or
nonequivalent ions causes a loss of the
two fold rotational axes and mirror
planes that are present in the Calcite
Group structure. Dolomite's symmetry
class is bar 3 whereas the Calcite
Group's symmetry class is bar 3 2/m. The
loss of symmetry allows only simple
crystal forms to be used by the Dolomite
Group minerals, mostly rhombohedrons.
Dolomite is a very common mineral and
ankerite is much more scarce. The other
members are considered rare to very
rare. The rarity of the members of this
group can be tied to the closeness in
radius of the A and B ions. In dolomite
the A and B ions are calcium and
magnesium which have the largest ionic
radius differential of the group
(approximately 33%). If the A and B ions
are close in radius, then they tend to
not segregate as easily into the
separate A and B layers, which is
required to form this structure and
therefore these minerals.
Minerals That Belong to the Dolomite Group
• Ankerite Ca(Fe, Mg, Mn)(CO3)2
• Benstonite (Ba, Sr)6(Ca, Mn)6Mg(CO3)13
• Dolomite CaMg(CO3)2
• Huntite CaMg3(CO3)4
• Kutnohorite Ca(Mn, Mg, Fe)(CO3)2
• Minrecordite CaZn(CO3)2
• Norsethite BaMg(CO3)2
The borate minerals nordenskoldine and
tusionite are isostructural with the
Dolomite Group minerals.
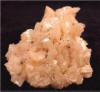
| Specimen: |
Cluster of curved, pink
Dolomtite crystals ("Pearl
Spar") |
| Locality: |
Picher, Ottawa Co., Oklahoma |
| Magnification: |
Specimen Grade |
| Collection: |
Hershel Friedman |
| Photograph: |
Hershel Friedman |
| Specimen: |
Curved white Dolomite crystals
with Chalcopyrite |
| Locality: |
Picher, Ottawa Co., Oklahoma |
| Magnification: |
+2 |
| Specimen Grade: |
B |
| Collection: |
Hershel Friedman |
| Photograph: |
Hershel Friedman |
Dolomite Industrial Use
A major source of magnesium,
particularly for agricultural and
pharmaceutical applications.
Dolomite General Use
Dolomite is used for manufacturing
certain types of refractory bricks used
in steel making. The dolomite is heated
to a high temperature to drive off the
carbonate as carbon dioxide and the
remaining material, a mixture of calcium
and magnesium oxides, is blended with
carbon and other materials and pressed
into blocks for the furnaces. The
magnesium and calcium oxides have very
high melting points and are an
excellent, inexpensive refractory
material.
Dolomite is also used as a source of
magnesium oxide for making magnesium
metal and for chemical uses, such as the
common laxative milk-of-magnesia.
Dolomitic limestone's and dolomites are
mined along with limestone and used for
crushed stone and aggregates for
manufacture of pavement, concrete for
construction and as fill material.
Dolomite is also used in some cement, as
a source of magnesium. Of course
Dolomite is also used as mineral
specimens.
Dolomite specimens from the Iran are
very popular among mineral collectors
and dealers. The clear transparent
specimens from Iran are rare and
unusual, and are in high demand by
Collectors.
Dolomite Rock is used as an ornamental
and structural stone, and for extracting
certain metals from their ores. It is
useful in the chemical industry in the
preparation of magnesium salts.
Recommended Filled of Application
| Kind of powder |
Talc |
Mica |
Kaolin |
Red Iron oxide |
Fluorine |
Dolomite |
Calcite |
Bentonite |
Barite |
| Ceramics |
• |
|
• |
• |
• |
• |
• |
• |
• |
| Chinaware |
• |
|
• |
|
|
|
|
|
|
| Excavation |
|
• |
|
|
|
• |
• |
• |
• |
| Elecrode |
• |
• |
|
|
• |
• |
• |
|
• |
| Feed |
|
|
|
|
|
|
• |
• |
|
| Glass |
• |
|
|
|
• |
• |
• |
|
• |
| Glaze |
|
|
• |
|
• |
• |
|
• |
|
| Glue |
• |
|
• |
|
|
|
• |
|
|
| Gerannlation (p.v.c) |
|
|
|
|
|
|
• |
|
|
| Insecticide |
• |
|
• |
|
|
|
|
• |
• |
| Isolation |
• |
|
|
|
|
|
• |
|
|
| lining |
|
|
|
|
|
• |
• |
|
• |
| Paint |
• |
• |
• |
• |
|
|
• |
• |
• |
| Pharmaceutical |
• |
|
• |
|
|
|
• |
• |
• |
| Plastic |
• |
|
• |
|
|
|
• |
|
• |
| Rulp & paper |
• |
|
|
|
|
|
• |
• |
• |
| Rubber |
• |
• |
• |
|
|
|
• |
|
• |
| Textile |
• |
|
|
|
|
|
• |
|
• |
|